HBV Reverse Transcriptase (HBV RT) plays a central role in the life cycle of the Hepatitis B virus, facilitating viral replication and presenting a critical target for antiviral treatments. As a key enzyme, understanding its structure and functionality can aid in developing effective therapeutics and improving management strategies for HBV infections globally.
Hepatitis B virus (HBV) is a significant global health concern, affecting millions worldwide and leading to severe liver diseases such as cirrhosis and hepatocellular carcinoma. A crucial component in the HBV life cycle is the HBV Reverse Transcriptase (HBV RT), an enzyme critical for viral replication.
This enzyme's role and functionality provide essential insights into HBV pathogenicity and are pivotal for developing antiviral treatments. The understanding of HBV RT's mechanics not only aids in grasping the intricacies of viral replication but also facilitates the design of therapeutic agents aimed at mitigating the effects of hepatitis B infection. By delving deeper into the biology of this virus and its replication processes, researchers can discover innovative solutions to combat the disease.
HBV Reverse Transcriptase is responsible for converting the viral RNA genome into DNA, a critical step that allows the virus to replicate and persist within the host. This enzyme operates through a complex mechanism involving multiple steps, including RNA-dependent DNA synthesis, RNA hydrolysis, and DNA-dependent DNA synthesis. Each of these steps is a potential target for antiviral drugs aimed at disrupting HBV replication.
During the replication process, HBV RT first synthesizes a negative-strand DNA from the RNA genome. Following this, the RNA strand is removed, and then a complementary positive-strand DNA is synthesized, resulting in the double-stranded DNA form of the virus that is fully integrated into the host's genome. This integrated DNA can persist for years and can lead to chronic infection, showcasing the importance of HBV RT in not just replication but also in the established long-term infection.
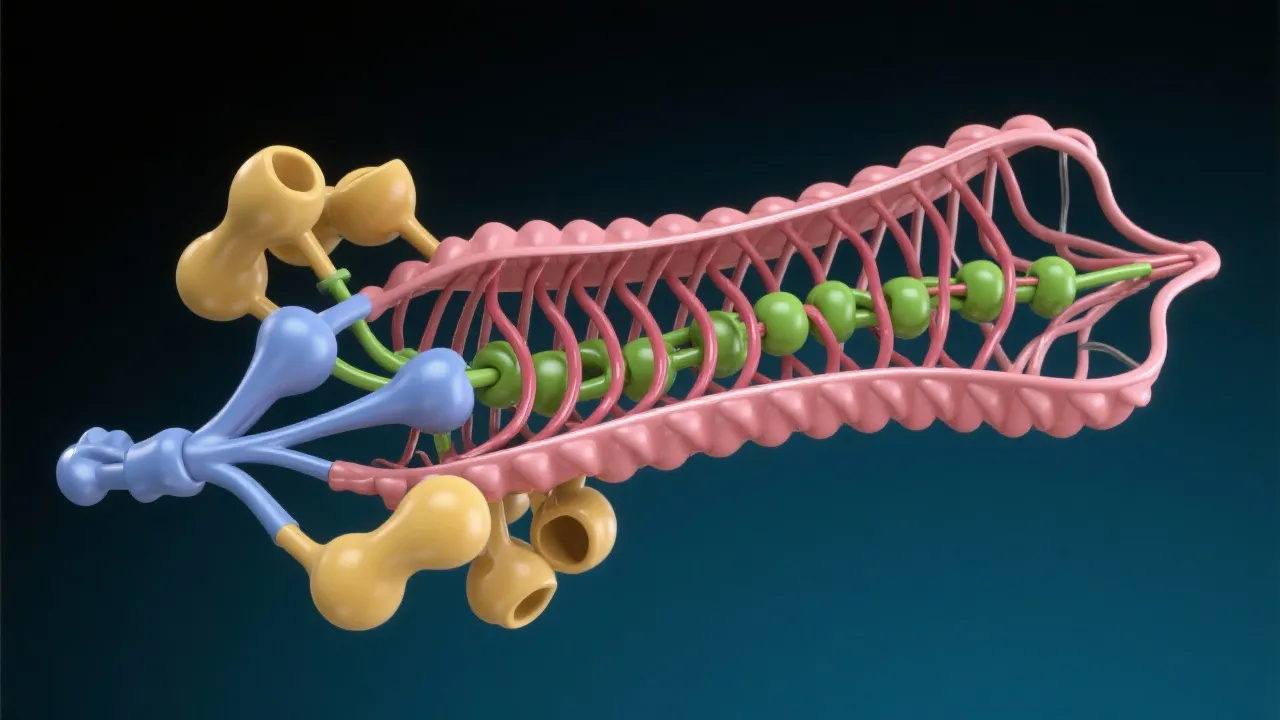
The structure of HBV RT is vital for understanding its function and the mechanisms of inhibition by antiviral drugs. This enzyme has distinct functional domains responsible for its various activities. Detailed structural studies using techniques such as X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy have shed light on its configuration, revealing potential sites for drug binding and inhibition.
The HBV RT consists of several domain regions, including a polymerase domain and a ribonuclease H (RNase H) domain. The polymerase domain is primarily responsible for the DNA synthesis process, while the RNase H domain plays a critical role in RNA degradation, ensuring that the RNA strand of the RNA-DNA heteroduplex is removed after the DNA strand has been synthesized. These structural characteristics not only elucidate the functional mechanisms of the enzyme but also provide a basis for the rational design of inhibitors that can effectively target specific domains to halt HBV replication.
Antiviral therapies for HBV typically target HBV RT to inhibit viral replication. Nucleoside and nucleotide analogs such as Tenofovir and Entecavir are commonly used drugs that mimic the natural substrates of the enzyme, thereby disrupting its activity. Despite the effectiveness of these treatments, good reliance can lead to drug resistance, necessitating continuous research into alternative therapeutic strategies.
In recent years, the combination of existing antiviral therapies has shown promise in increasing treatment efficacy while minimizing resistance. By using different mechanisms of action, combination therapy can better overcome the virus's ability to adapt and evolve, potentially leading to improved long-term outcomes for patients with chronic hepatitis B.
| Drug | Mechanism of Action | Advantages | Challenges |
|---|---|---|---|
| Tenofovir | Disrupts DNA synthesis by mimicking natural substrates | High efficacy and safety profile | Potential for kidney dysfunction with prolonged use |
| Entecavir | Inhibits both priming and synthesis stages | Lower risk of resistance development | Requires careful dosing in patients with renal impairment |
| Adefovir | Competitively inhibits the viral reverse transcriptase | Effective in patients with resistance to other antivirals | Risk of nephrotoxicity and limited antiviral potency |
| Lamivudine | Inhibits reverse transcription and DNA synthesis | Well-tolerated with low rate of side effects | High potential for developing resistance |
The ongoing research aims to overcome the limitations of current HBV treatments such as drug resistance and side effects. Novel inhibitors targeting different stages of HBV RT or additional viral replication mechanisms are in development. Combination therapies offer another promising avenue, potentially enhancing antiviral efficacy and reducing resistance emergence.
Additionally, emerging research is focusing on host-targeted therapies that modulate the immune response to HBV infection, aiming to enhance the body’s ability to fight the virus. Therapeutics in this category could include immune modulators or therapeutic vaccines designed to boost the host's immunity against HBV. These approaches may represent a paradigm shift in how hepatitis B is treated, transforming management strategies from purely antiviral therapies to comprehensive immune-based interventions.
Moreover, significant advancements in genomics, proteomics, and bioinformatics are leading to a better understanding of HBV host interactions, which could unveil new therapeutic targets. By identifying specific host cellular pathways and proteins that HBV exploits for replication and evasion of the immune response, researchers could create novel treatment strategies that interfere with these interactions.
Q: What makes HBV Reverse Transcriptase a significant target for drug development?
A: As a crucial enzyme in HBV replication, targeting HBV RT can effectively disrupt viral reproduction, making it a central target for antiviral therapies.
Q: Can resistance to HBV RT inhibitors develop?
A: Yes, resistance can develop with prolonged use of current HBV RT inhibitors, presenting challenges in good HBV management. This necessitates the development of novel therapies and combination treatments to stay ahead of the virus's adaptability.
Q: How do current inhibitors of HBV RT work?
A: Current inhibitors, such as nucleoside and nucleotide analogs, work by mimicking HBV RT's natural substrates, which disrupts the viral replication process at various stages, preventing the virus from effectively propagating.
Q: What are the potential side effects associated with HBV RT inhibitors?
A: Side effects may vary among different drugs, but common issues include gastrointestinal disturbances, fatigue, headache, and specific drug-related complications such as renal dysfunction with Tenofovir or lactic acidosis with NRTIs.
The HBV Reverse Transcriptase remains at the forefront of hepatitis B research and treatment strategies. Continued innovation in drug development can significantly enhance treatment outcomes and improve quality of life for individuals impacted by HBV infection globally. Ongoing clinical trials are essential to establish the efficacy and safety profiles of new agents while also determining the best combinations of existing treatments tailored to patient-specific needs, creating a multifaceted approach to managing this chronic viral infection efficiently.
Explore the Tranquil Bliss of Idyllic Rural Retreats

Ultimate Countdown: The 20 Very Legendary Gaming Consoles Ever!

Understanding Halpin and its Influence

Affordable Full Mouth Dental Implants Near You

Discovering Springdale Estates

Illinois Dentatrust: Comprehensive Overview

Embark on Effortless Adventures: Unveiling the Top in Adventures Made Easy Outdoor Equipment

Unveiling Ossur Valves: Innovation in Prosthetics

Unlock the Full Potential of Your RAM 1500: Master the Art of Efficient Towing!
